Disclaimer: This information has purely educational and scientific intend.
Often Potassium nitrate is not available or is too expensive so I decided to describe an economically viable way of synthesizing KNO3 from cheap and available resources. The process is not difficult, requires no specialized chemicals and the yield is excellent. Oversimplified description would be:
Calcium Nitrate + Potassium Chloride = Potassium nitrate + Calcium Chloride
In reality things are a bit more complicated, but nothing to worry.
MATERIALS
Potassium Chloride (KCl)
The best source of cheap and very pure Potassium Chloride is salt for water softener installations. It is sold on many places in different sizes – just make sure you are getting Potassium and not Sodium chloride softener. Don’t bother buying table salt replacement as it is too expensive and very impure.
Calcium Nitrate (Ca(NO3)2)
Calcium Nitrate is sold as fertilizer in most hydroponic shops and agriculture suppliers in different bag sizes. In reality it is not pure Calcium Nitrate but rather a Calcium-Ammonium nitrate decahydrate [NH4(NO3)*5Ca(NO3)2*10H2O], which means that it contains Calcium nitrate, ammonium nitrate and water. What we are interested is the nitrate ion (ammonium or calcium both work).
Because of the presence of ammonium nitrate we will get additionally some ammonium chloride, but this doesn’t really change anything for us. Hence a proper description of the ion-exchange reaction would be:
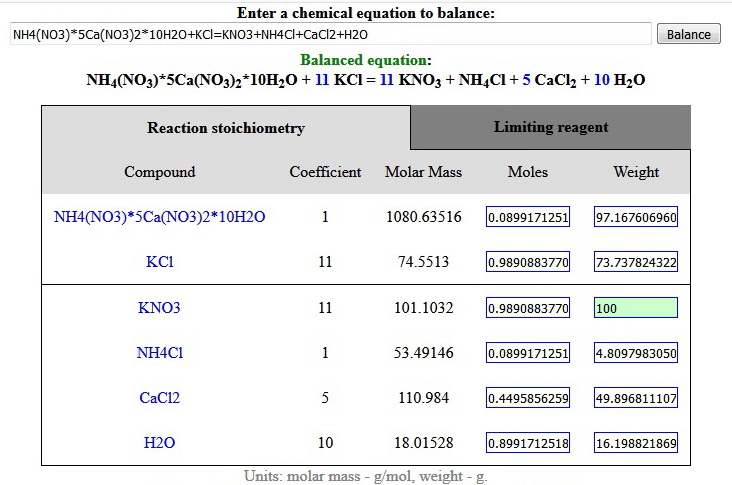
NH4(NO3)*5Ca(NO3)2*10H2O + 11KCl = 11KNO3 + NH4Cl + 5CaCl2 + 10H2O
To help you calculating the proper weights that you need instead of playing with molar masses you can use the following on-line chemistry calculator (just click on the picture) where you can enter the weight of one of the input reagents or desired weight of the reaction products and the rest will be automatically calculated. The corresponding solubility can be found in the Solubility table.
Basically we dissolve the reagents in hot water, mix them to allow the ion exchange and cool down the solution to obtain the desired product (KNO3) which will crystalize in the reaction vessel since KNO3 has much lower solubility than the other products.
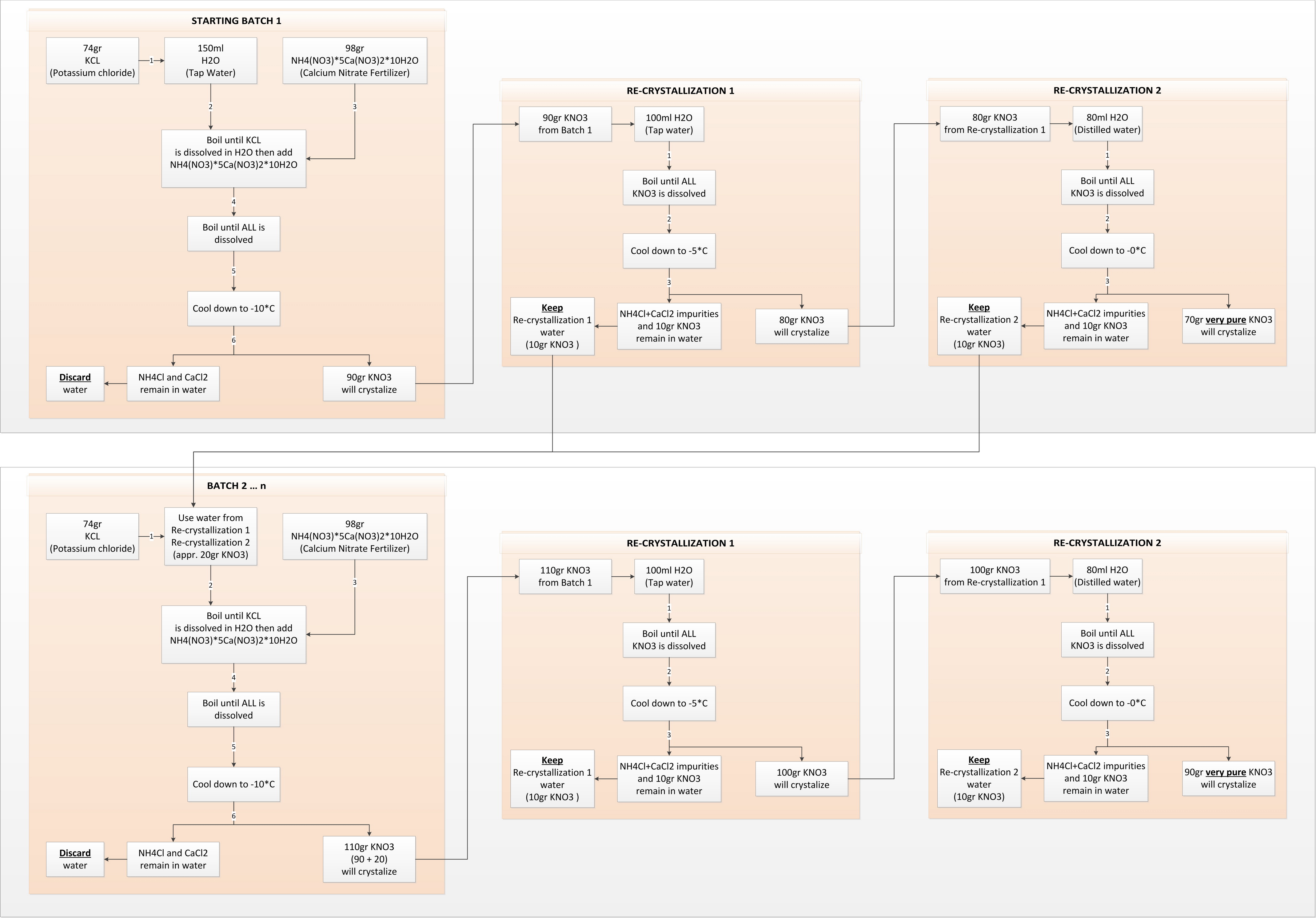
In order to finish with high purity KNO3 we need to do preferably 2 re-crystallizations of the Potassium nitrate. However every time we do that inevitably some KNO3 remains dissolved in the water, which lowers our final yield. To keep the final yield high we need to work in batches to minimize the water used and recycle it while “playing” with the solutions temperatures depending on the values from the Solubility table.
 STEP1 – BATCH 1 – dissolve 74gr Potassium chloride in 150ml boiling tap water, it takes some time to fully dissolve.
STEP1 – BATCH 1 – dissolve 74gr Potassium chloride in 150ml boiling tap water, it takes some time to fully dissolve.
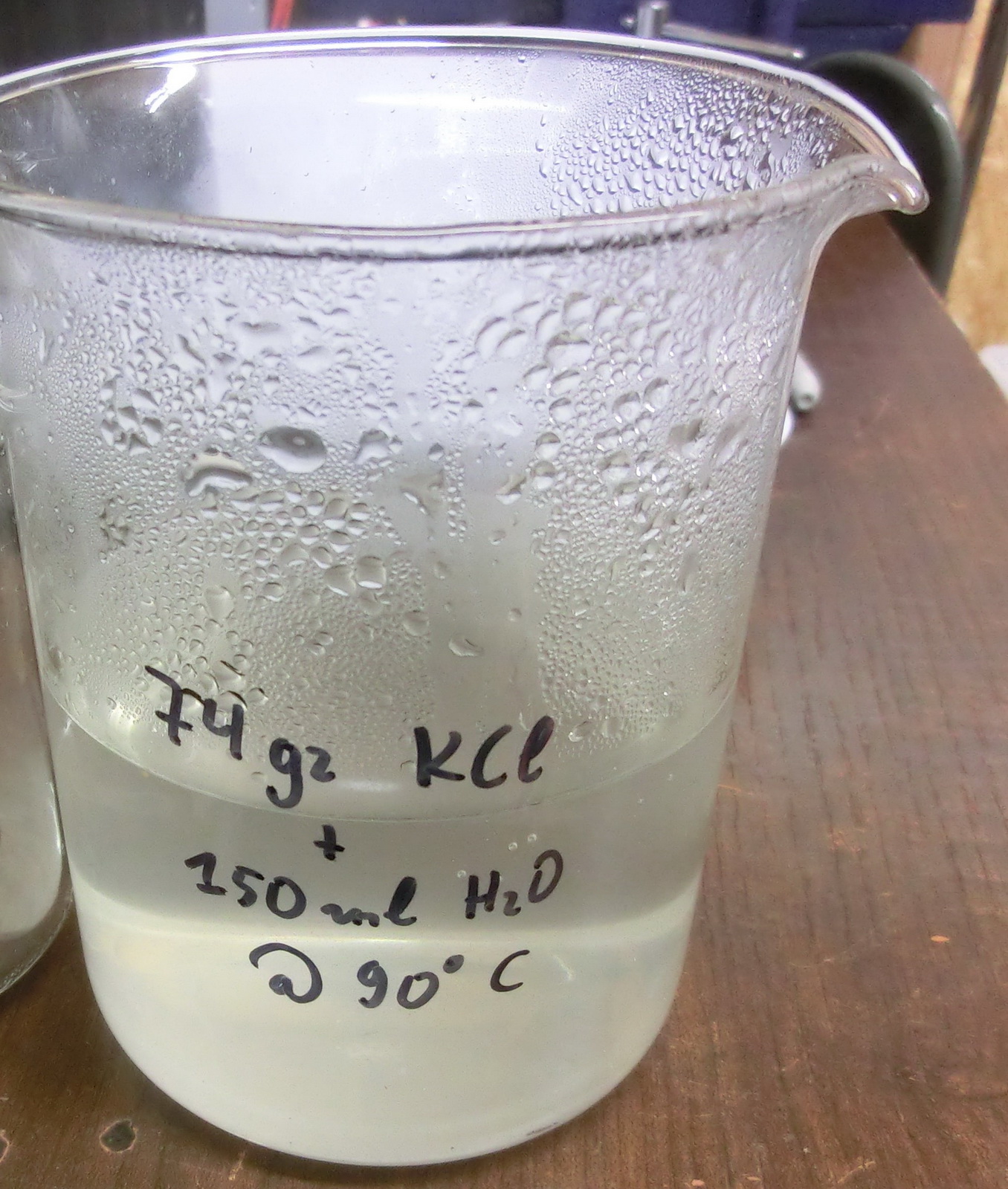 STEP2 – to the boiling solution add 98gr Calcium nitrate (calcium-ammonium decahydrate) and stir until all is dissolved. 100gr of KNO3 will be formed.
STEP2 – to the boiling solution add 98gr Calcium nitrate (calcium-ammonium decahydrate) and stir until all is dissolved. 100gr of KNO3 will be formed.
 STEP3 – cool down to (negative) -10*C – no point of further cooling it down because you will get water crystals. About 90% of the KNO3 (90gr) will crystalize, however contaminated with Calcium chloride and Ammonium chloride. Discard the remaining water.
STEP3 – cool down to (negative) -10*C – no point of further cooling it down because you will get water crystals. About 90% of the KNO3 (90gr) will crystalize, however contaminated with Calcium chloride and Ammonium chloride. Discard the remaining water.
 STEP4 – dissolve the 90gr KNO3 from STEP3 in 100ml boiling tap water for first re-crystallization.
STEP4 – dissolve the 90gr KNO3 from STEP3 in 100ml boiling tap water for first re-crystallization.
STEP5 – cool down to (negative) -5*C. About 90% of the KNO3 will crystalize giving appr 80gr relatively pure KNO3. Keep the remaining water, which contains about 10gr dissolved KNO3 to be used in Batch 2.
STEP6 – dissolve the 80gr KNO3 from STEP5 in 80ml boiling distilled water for second re-crystallization.
STEP7 – cool down to 0*C. About 90% of the KNO3 will crystalize giving appr 70gr very pure KNO3. Keep the remaining water, which contains about 10gr dissolved KNO3 to be used in Batch 2.
STEP1 – BATCH2 – repeat as in Batch1 but now instead of tap water, for this step use the water from the two re-crystallizations in the previous Batch.
STEP2 …. STEP7 – repeat as in Batch 1.
…
Normally if we cool water solution of KNO3 below 0 degrees it will start forming water crystals together with the KNO3 crystals but because the solution in STEP3 of each batch contains high amount of Calcium Chloride we can cool it down to -10 degrees without forming water crystals which provides few additional percent to the final yield of KNO3. Additionally the remaining dissolved KNO3 from the both re-crystallizations in the preceding batch is carried on to the next batch and recuperated. This gives a final yield of about 85-90 percent of very pure KNO3.
The following diagram describes the “near ideal” process. If you want to scale it up (or down) you have to prorate all amounts. The temperatures and quantities of water are not critical as long as all reagents gets dissolved. More water and higher temperatures during the KNO3 crystallization will leave more KNO3 in the solutions.
Finally if you want really pure KNO3 after drying the crystals you can wash them with pure denatured Ethanol sold in most hardware stores. Potassium Nitrate is insoluble in ethanol while Calcium Chloride and Ammonium Chloride are somewhat soluble. After washing the KNO3 you can recycle the Ethanol by distilling it – the dissolved chlorides will remain in the distilling vessel. However in most cases this will be an “overkill”.
Every time when you separate the KNO3 crystals from the liquor it is strongly advisable to remove (not evaporate) as much liquor from the crystals as possible. Remember that all contaminants (calcium and ammonium chlorides) remain in the liquor and if you leave wet crystals then you leave also some of the chlorides at the crystal’s surface –this makes the Potassium nitrate more hygroscopic.
Removing the liquor is best done with centrifuges or vacuum filters. The easiest way is DIY vacuum filter (courtesy of good friend) made from an old vacuum cleaner, a small bucket and a coffee filter – like the one that I am using shown on the picture. It is a good practice during vacuum filtering to rinse the crystals by spraying some water over the crystals.
As always the best time to do the conversion is Winter! All heat from the appliances remains inside and outside the chill doesn’t cost anything – almost no electricity is wasted.





Hi Dyanko,
Nice to see you keeping busy. Are you making KN03 for rocket fuel? What is the cost of doing it this way?
Hello Charlie,
the final KNO3 is of sufficient purity to be used in sugar motors. As for the cost – it really depends on your local market supply. In some instances it could be significantly cheaper – you have to compare prices and availability in your region.
will Epson salt work for this??
Epsom salt (Magnesium Sulphate) can potentially “donate” only Magnesium and Sulphate ions – thus, No it can’t be used for making Saltpeter (Potassium Nitrate).
thought so also will this salt work https://www.woolworths.com.au/shop/productdetails/35622/diet-rite-lite-salt
It can be used.
However as I wrote in my article “Don’t bother buying table salt replacement as it is too expensive and very impure.”
I did small test got crystal growth but took a long time could that because its very impure or that my freezer isn’t cold enough.
Probably the Potassium Chloride in the reaction was less than it should have been, hence the low yield.
If you check the contents of the earlier mentioned salt, you will see that probably half of it is Sodium Chloride (common table salt).
Please Answer me..
Will this Fertilizer Work as Calcium nitrate?
26% ( CaO )
19% ( Ca )
15% Total Nitrogen
13.50% N-NO3
1.50% N-NH4
—————————-
Please answer me as soon as possible Because i’m ordering it now from the internet 🙂
Calcium oxide (CaO) will be a problem.
I made it but The potassium nitrate was only 37grams..
(Sorry for my bad English)
The lower yield is because of the Calcium oxide (CaO) – you need to recalculate the Potassium Chloride and Calcium Nitrate proportions taking in consideration that portion of the Potassium Chloride will react with the Calcium Oxide in the fertilizer too.
Hmmm.. Can you please recalculate it for me because I don’t know how to?? i will be so thankful 🙂
These are simple chemical reactions. For balancing the equations, as well for calculating the stoichiometric quantities you can use the web link I provided in this article.
Try first to figure it out on your own, Internet is full of information how to do basic chemical calculations. It will be very helpful and educational.
Ok thank you… but I searched and found nothing
You can use the link that I posted in the article for balancing the equations and calculating the stoichiometric quantities.
Nevertheless for maximum yield you have to do a multistage re-crystallization as indicated in the flowchart, however the entire flowchart needs to be recalculated taking in consideration the Calcium Oxide and the additional products of the reaction.
Yea i understand what did you say but The problem is that i don’t know how to recalculate taking in consideration the Calcium Oxide and the additional products of the reaction.
this is my problem… I’m not an expert and thank you so much for your time you’re so helpful
Hello, I’m from China. Glad to see your beaker from China. Due to Chinese laws and regulations, we cannot buy any potassium nitrate. But calcium nitrate is sold in the store. Can I use potassium carbonate and calcium nitrate to make it?
As a supplement, I can buy pure Ca (NO3) 2 * 4H2O
Yes, the reaction is:
K2CO3 + Ca(NO3)2 = 2 KNO3 + CaCO3
You can use this equation and the link from my post to calculate the stoichiometric quantities.
However very fine Calcium Carbonate (CaCO3) will precipitate and has to be filtered out from the solution, which could be problematic. After that the solution will contain mostly KNO3 which can be let to crystallize from a supersaturated solution.
How do you calculate how much hot water should bee used. Why 150 ml and not e.g 200 ml
Hello,
You calculate it based on the solubility of the salts and the temperature.
Hi excuse me, is it normal for the solution become smoothie like in step4? Thank you for your reply
Hi,
probably fine Calcium Carbonate is crystalizing with the temperature increase.
http://www.blog.exrockets.com/blog/making-potassium-nitrate-from-calcium-nitrate-and-potassium-chloride/#comment-6846
I’m assume every instance of “gr” is gram, not grain?
probably should use the right units
Indeed, “gr” stands for grams.
Hello sir,
I mix 436 gram potasium chloride with 900 ml water after dissolved all KCl, mix calcium ammonium nitrate fertilizer 574 gram to the solution. After dissolved all solids, i filter the solution and place the solution in the freezer. But i will get 300 gram crystal only. How to increase my yield of potasium nitrate? Reaction time affect yield percentage of product? Heat the solution in slow flame in 1 hr still all solid dissolved is increasing yield percentage?
hi
I have similar situation but one exception
I have pure calcium nitrate with no Ammonium nitrate
How much water I should add at the beginning for 2KCl+Ca(NO3)2=2KNO3+CaCl2 ?
Hi Pinko,
Im curious, if I use a fertilizer with the following compounds:
KNO3 : ~35%
NH4NO3: ~25%
NH4Cl: ~15%
Would the reaction then be NH4(NO3)*KNO3*NH4Cl+KCl=KNO3+NH4Cl , KNO3 is obviously already a part of the precusor, but I figured KCl could be added in lesser quantity to convert the ammoniumnitrate. Im guessing fractional crystallization can still be used to remove NH4CL, even though the amount to remove will be a bit larger.
Just add potassium hydroxide or carbonate to saturated solution to decompose ammonium nitrate then when solution is clear let it cool to crystallize crude potassium nitrate and purify